Who Are The Biggest Fraudsters In Minnesota (And America)? They're Not Somali
It’s been seven weeks since the Department of Justice stepped up its investigation of fraud at Minnesota social service organizations run by citizens of Somali descent. The Department of Homeland Security simultaneously stepped up the Immigration and Customers Enforcement (ICE) presence in the Twin Cities.
The results thus far? One dead, one wounded, and the entire Somali community living in fear after being demonized by President Trump with statements like “we don’t want them in our country” and “they should go back to where they come from.”
A horrified public has rightfully focused on the murderous tactics and egregious civil rights violations perpetrated by out-of-control ICE agents. Nightly newscasts are filled with scenes of masked, camouflaged, machine gun-toting men patrolling the streets of Minneapolis; making warrant-less stops of anyone with brown skin; arresting those not carrying proof of citizenship; and throwing tear gas canisters at peaceful demonstrators. They’ve even entered a hospital and shackled a severely injured patient to his bed. The 2,000-plus ICE agents now in the city is nearly four times the size of the local police force.
Why are they there? The Trump administration was given cover to step up its attacks on the Somali immigrant community by national media coverage of longstanding fraud investigations in the state. The Minnesota Attorney General has issued three indictments over the past two years, while the U.S. Attorney in Minnesota brought one, all begun during the Biden years.
In the wake of its latest incursion, the administration announced it would block all federal social service funding to five blue states including Minnesota, a move that was temporarily blocked in a New York federal court earlier this month.
Media attention drove events
The national media got the ball rolling in late November when a story in the New York Times reported on three fraud schemes at programs that feed the hungry and provide day care, allegedly costing taxpayers over $1 billion. The three cases mentioned totaled a third of that amount. A key paragraph near the top claimed “Minnesota’s fraud scandal stood out even in the context of rampant theft during the pandemic, when Americans stole tens of billions through unemployment benefits, business loans and other forms of aid, according to federal auditors.”
The link (which was included in the original Times story) leads to a Government Accountability Office report that estimated there was at least $100 billion in pandemic-era unemployment insurance fraud. The GAO blamed all 50 states for failing to police the program. That level of fraud would be 400 times greater than the largest fraud scheme so far confirmed in a Minnesota court room.
Meanwhile, the Trump administration is paring back fraud enforcement in red states. Last October, it put on hold a Biden-era order that Mississippi repay $101 million for welfare embezzlement. Agency officials — not patients, not providers — had involved former professional football quarterback Brett Favre in a scheme to channel temporary assistance families money into a fund for building a volleyball stadium on the University of Southern Mississippi campus.
The Times followed up two weeks later with coverage of a press conference held by acting U.S. Attorney Joseph H. Thompson, who was appointed by Trump last June. He announced a new probe of 14 Medicaid-funded programs for suspicious billing practices. Half of the $18 billion spent by those programs since 2018 was stolen, he said, although no specific allegations were included. “What we see in Minnesota is not a handful of bad actors committing crimes,” he said. “It is staggering industrial-scale fraud.”
Then, the day after Christmas, a conservative YouTube influencer named Nick Shirley posted a widely-seen video highlighting shuttered day care centers and Somali daycare workers refusing him entry. A few days later, Homeland Security secretary Kristi Noem, whose department has no jurisdiction over the allegedly defrauded programs, called for a “massive investigation of daycare and other rampant fraud” and unleashed ICE agents to begin investigating sites based on tips from the YouTube video, not FBI investigators, according to CBS News.
There is no doubt greedy operators ripped off Minnesota safety net programs. Several of the nearly 100 people under investigation have already pleaded guilty. Democratic Gov. Tim Walz, who dropped out of his re-election campaign in the wake of the scandals, clearly was slow to heed warnings from local and federal investigators about the large fraud schemes in the state’s programs.
Who’s the biggest alleged fraudster in Minnesota?
But if federal officials in Minnesota really want to go after industrial-scale fraud, they ought to step up their slow-motion investigation of UnitedHealth Group, the nation’s largest health insurer, whose headquarters just happens to be in Minneapolis.
They could start by taking a look at the UnitedHealth Group Abuse Tracker run by the American Economic Liberties Project, a anti-monopoly watchdog organization founded by Fordham Law School professor Zephyr Teachout. UHG, according to the tracker, has been accused of myriad wrongdoings in recent years, including:
- Twelve reports and five lawsuits for upcoding and overbilling the federal government;
- Three reports and one lawsuit for violating patient privacy;
- Fifteen reports and five lawsuits for denying patient care based on cost instead of medical necessity;
- Fourteen reports and seven lawsuits for steering patients and providers toward UHG owned subsidiaries in order to increase company profits; and
- Eight reports of corrupt practices.
UHG, in its responses to news organizations and in court filings, denied every finding and claim, including those in last week’s report from Sen. Chuck Grassley’s office. After reviewing 50,000 internal documents subpoenaed by the Judiciary Committee, the report found UnitedHealthcare, UHG’s insurance arm, maintained a huge workforce dedicated to inflating risk-adjustment codes on its 8 million Medicare Advantage customers. This upcoding allegedly bilks the government of billions of dollars annually.
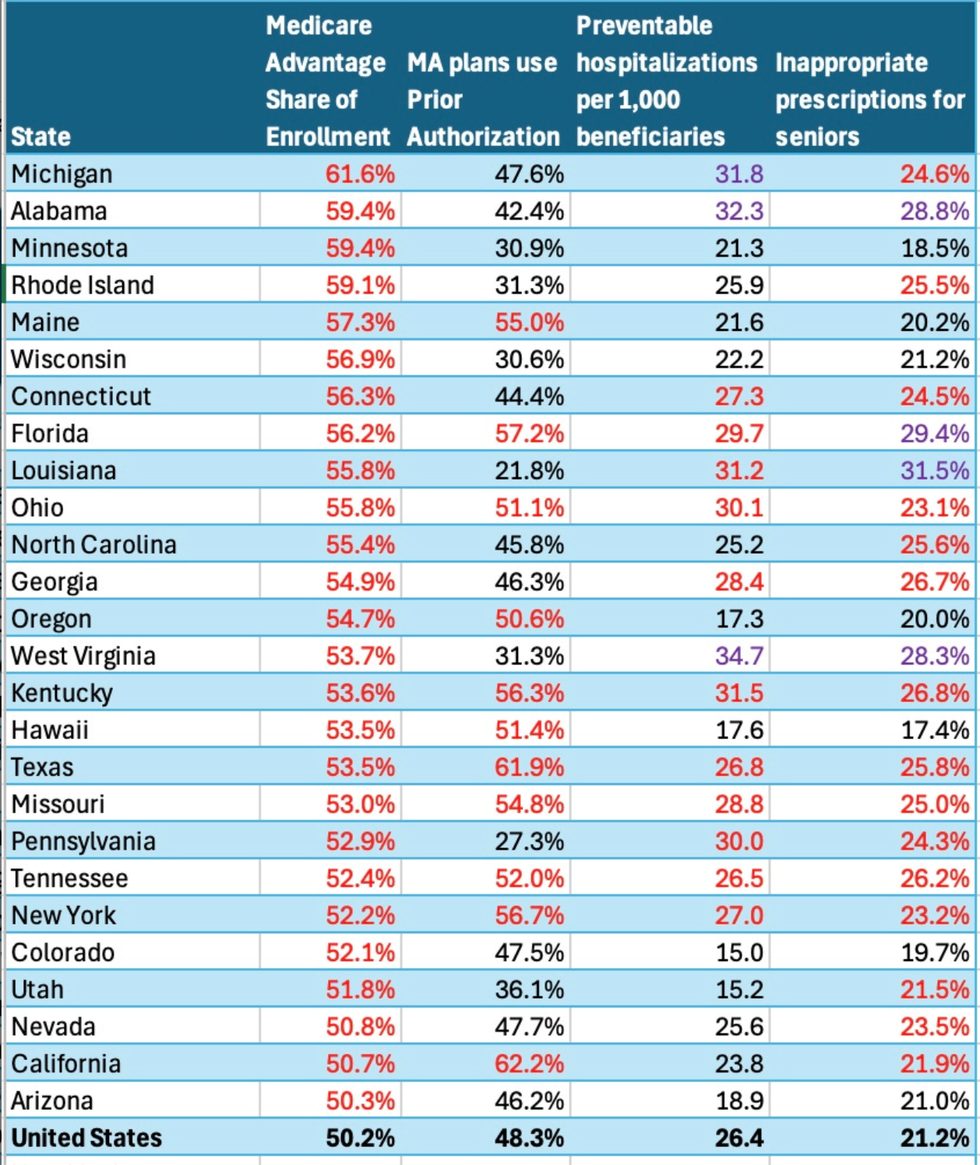
Outside analysts and the Medicare Payments Advisory Commission have repeatedly accused private insurers of overcharging the Centers for Medicare and Medicaid Services (CMS)’ MA program, which now covers over half of all seniors. The most recent estimates suggest over-billing based on upcoding needlessly costs taxpayers $84 billion a year.
Yet federal prosecutors bungled the one whistleblower case that finally came to trial after a decade of legal maneuvering. A special master ruled last February that the Department of Justice had failed to prove the insurance giant deliberately exaggerated how sick its Medicare Advantage patients were to increase federal reimbursements.
But there are still numerous cases pending against UHG and other MA providers. Multiple investigations have been announced by the DOJ. Just last week, Kaiser Permanente, a leading Medicare Advantage insurer in California, agreed to pay $556 million to settle claims it bilked Medicare of $1 billion through upcoding between 2009 and 2018. The case took years to make its way through the courts.
Fraud is widespread
If one needs more evidence that the fraud uncovered in Minnesota is not out of line with typical health care and social service fraud schemes across the country, one need only look at the settlements in cases compiled by Bass, Berry & Sims, a law firm with an extensive practice defending corporate clients against False Claims Act suits. (The False Claims Act is a Civil War-era statute that allows whistleblowers and their lawyers to keep as much as a third of money recovered from firms convicted of defrauding the federal government.)
In just the first half of last year:
- Walgreens agreed to pay at least $300 million to resolve allegations its stores illegally filled invalid prescriptions for opioids and other controlled substances that were reimbursed by federal health care programs.
- Gilead Sciences agreed to pay $202 million to resolve allegations that it funneled kickbacks in the form of speaker fees, costly meals, and travel expenses to physicians to induce them to prescribe its HIV medications.
- California-based Seoul Medical Group and an affiliated radiology practice agreed to pay $62 million to settle claims it fraudulently increased Medicare Advantage reimbursements by falsely claiming patients had a severe spinal condition.
- A Pfizer subsidiary agreed to pay nearly $60 million to resolve allegations that it provided remuneration to physicians in the form of speaker honoraria and lavish meals in order to induce prescriptions of its migraine medication.
- Fresno-based Community Health System agreed to pay $31.5 million to settle allegations that it paid bonuses to physicians and subsidies for electronic health record systems in exchange for referrals and subsidies. The alleged illegal inducements included providing referring physicians with expensive meals, alcohol, and cigars provided in a lounge on premises at the health system.
- New York’s St. Vincent Catholic Medical Centers agreed to pay $29 million to resolve allegations that it kept the money despite learning that it had overcharged the Department of Defense for health care provided retired military members and their families.
- C.R. Bard Inc. and its affiliates agreed to pay $17 million to resolve allegations that they provided free samples and discounts to urology practitioners in a kickback scheme aimed at inducing use of the company’s catheters.
- And, last June, in Minneapolis, NUWAY Alliance, a substance use disorder treatment provider, agreed to pay $18.5 million to resolve allegations that it double-billed for treatment services and paid Medicaid patients to seek outpatient care.
The last case, the only one settled in Minnesota in the first half of last year, involved a non-profit organization whose last federal tax filing showed $28 million in annual expenses. Its CEO, David Vennes, earned $619,000 in 2024. None of the 12 high-paid executives and 8 board members listed on the non-profit’s 990 form have Somali last names.
During last year’s second half, the U.S. Attorney’s office in Minnesota indicted eight Somali residents for stealing millions of dollars from the state’s housing stabilization fund. How much was siphoned from the $300 million in grants made to their organizations from that fund since 2000 was not specified in the press release, but it would probably fall within the lower range of thefts that make various organizations’ tracker lists.
It’s not the immigrants; it’s not the poor
So is Minnesota a hotbed of fraud compared to other states? Does it call into question, as other media accounts have suggested, the very idea that a more generous safety net like the one in that state invites fraud?
No, sadly, the problem of fraud is the same there as it is everywhere, even in redder-than-red places like Mississippi. It is as American as apple pie (it’s the government’s money, so it’s nobody’s money). It is inadequately policed by federal and state officials, Democrats and Republicans alike.
The nation’s tattered social safety net, under assault by the Trump administration and shrinking daily, remains prone to abuse by unscrupulous operators. Medicare and Medicaid are especially juicy targets. Most of the perpetrators are lodged within large corporations run by white executives with excellent and expensive legal representation.
A real crackdown on fraud would go after those big fish first.
Merrill Goozner, the former editor of Modern Healthcare, writes about health care and politics at GoozNews.substack.com, where this column first appeared. Please consider subscribing to support his work.
Reprinted with permission from Gooz News






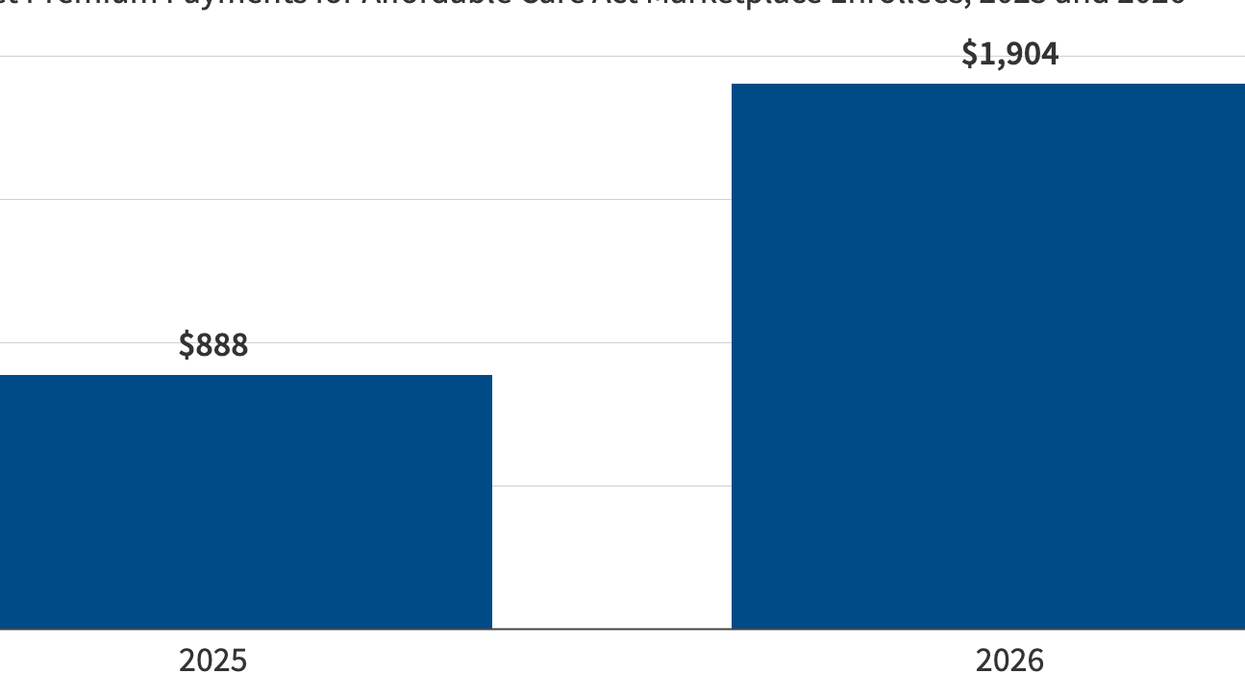


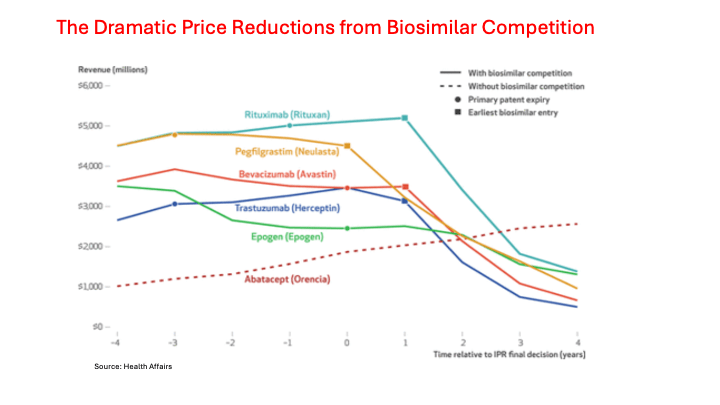
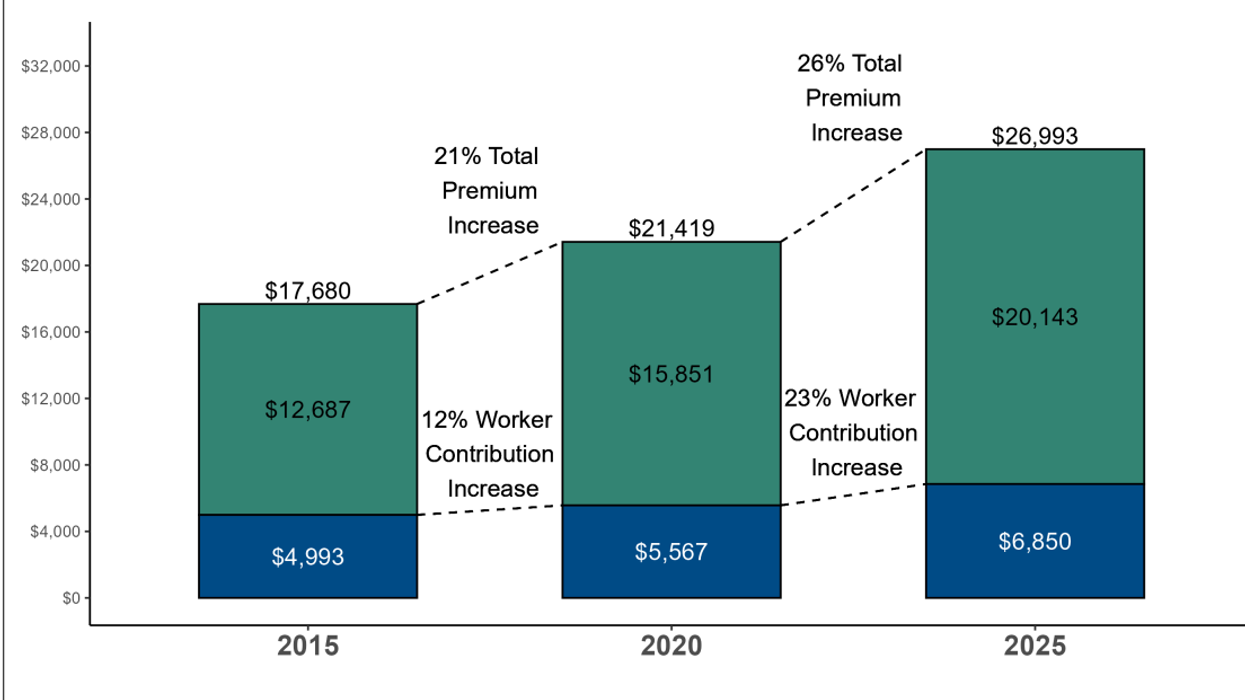
 Source: Kaiser Family Foundation
Source: Kaiser Family Foundation

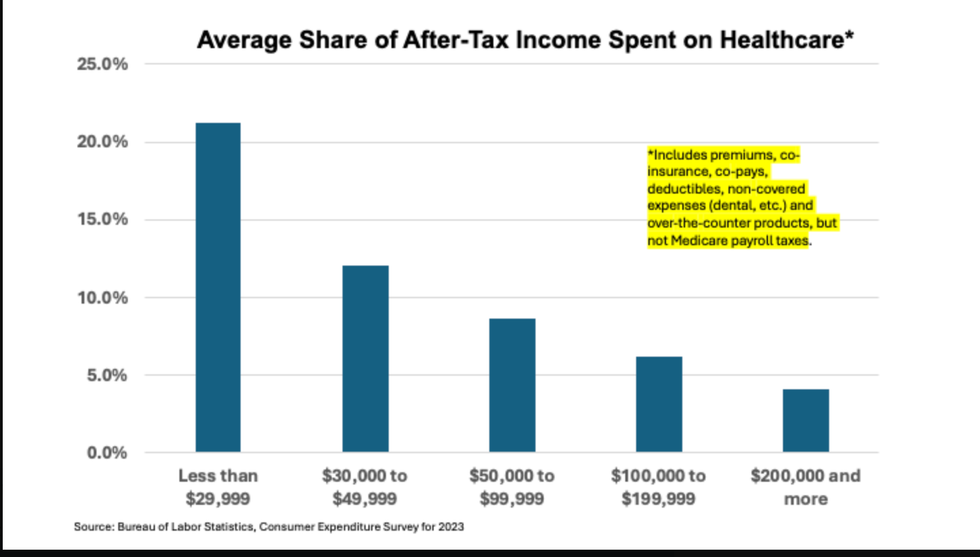



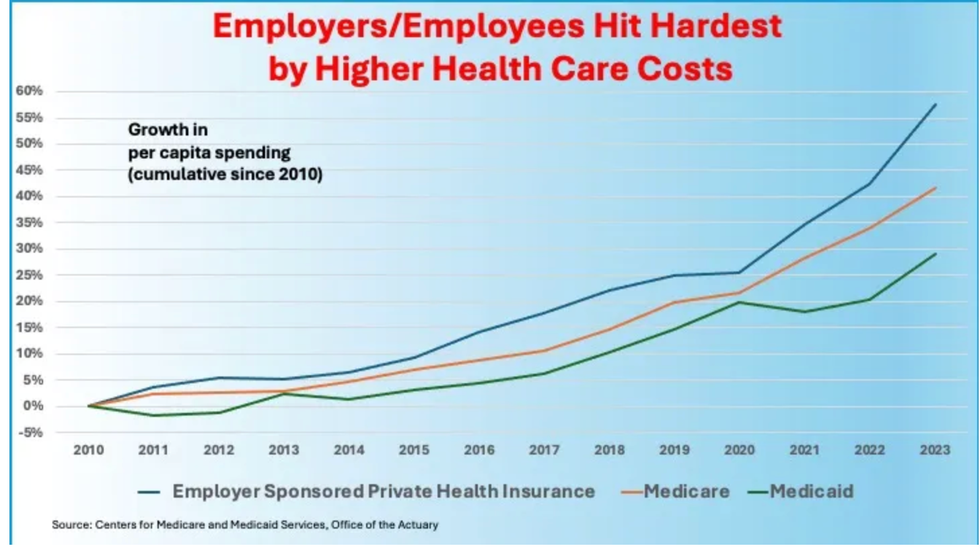
 Source: Atlanta Federal Reserve
Source: Atlanta Federal Reserve